The European Court of Justice (CJEU) has held that a parallel importer of wound dressings was free to add a small label to the outer packaging of the goods without infringing the brand-owner’s trade mark. It follows from this that the parallel trader was not obliged to notify the brand owner of the modified packaging as is usually required in pharmaceutical repackaging cases.
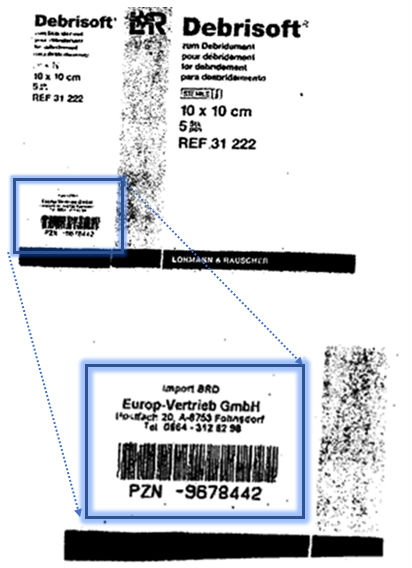
The label in question was stuck onto an unprinted space on the package (pictured below) and showed the name, address and telephone number of the importer and a barcode. It did not obscure the original brand name. The wound dressings remained in their original internal and external packaging. All the importer had done was to add the small label; it had not, for example, opened the box and inserted a translated patient information sheet.
Background
Under EU rules on the free movement of goods parallel traders are in principle free to buy branded pharmaceutical products in one EU Member State and sell them in a more expensive Member State. However, if the trader repackages the goods, which may be necessary to meet local requirements, strict conditions (sometimes referred to as the BMS conditions) must be complied with to avoid infringement of the originator’s trade mark. CJEU case law establishes that sticking a label onto the original outer packaging may constitute repackaging in this context. The BMS conditions have been developed in the case law and include, among other things, obligations to preserve the quality and safety of the goods and to avoid damaging the reputation of the brand-owner by ensuring that the new packaging is neat and of good quality. In addition, the trader must notify the trade mark owner before the repackaged products are put on sale and supply a sample of the modified packaging on demand, which gives the brand owner the opportunity to raise objections before the modified packaging goes onto the market.
Do the BMS conditions apply to medical devices?
The German court making the reference asked the CJEU whether the BMS conditions, which have been developed in relation to pharmaceutical products, apply to medical devices such as the wound dressings in this case. The CJEU did not answer this question specifically, although the case law suggests that this may be the case in some circumstances. Instead, the court focused on the nature of the label. It indicated that in a case such as this the BMS conditions would not be engaged in any event. The Court’s reasoning was that, unlike more extensive modifications to the packaging, a label of this kind simply giving the details of the importer, where the box had not been opened, did not pose a risk to the trade mark’s ability to indicate the origin of the goods. An important factor here was that the presence of the label would not suggest to customers that the goods had been interfered with by a third party at an earlier stage of their marketing.
Implications of the case
The case will be welcomed by parallel importers both of pharmaceuticals and medical devices, because it confirms that adding a label does not always require notification to the trade mark owner – key factors will be whether the box has been opened, the size and placement of the label, the content of the label – here it just gave details of the importer, whether it obscures the trade mark and whether it introduces any confusion as to origin, for example because of the way the importer’s trade mark is presented.
Case: Junek Euro-Vertrieb GmbH v Lohmann & Rauscher International GmbH & Co. KG, 17 May 2018 C-642/16
 Charlotte Tillett
Charlotte Tillett